Clinical trials are under tremendous pressure. Over the past decade, protocols have become more complex, regulatory requirements have tightened, and the cost of bringing a new drug to market continues to rise. At the same time, alternative approval pathways are becoming increasingly important- in 2024, 14% of new drugs were approved through the FDA’s Accelerated Approval pathway (NIH).
For sponsors and research teams, these dynamics create significant challenges. Traditional planning methods, i.e., manual feasibility assessments, fragmented data review, and reactive diversity tracking are no longer sufficient. Artificial intelligence (AI) is emerging as a strategic partner, helping trial planning become more predictive, efficient, and data-driven.
Why Current Approaches Fall Short
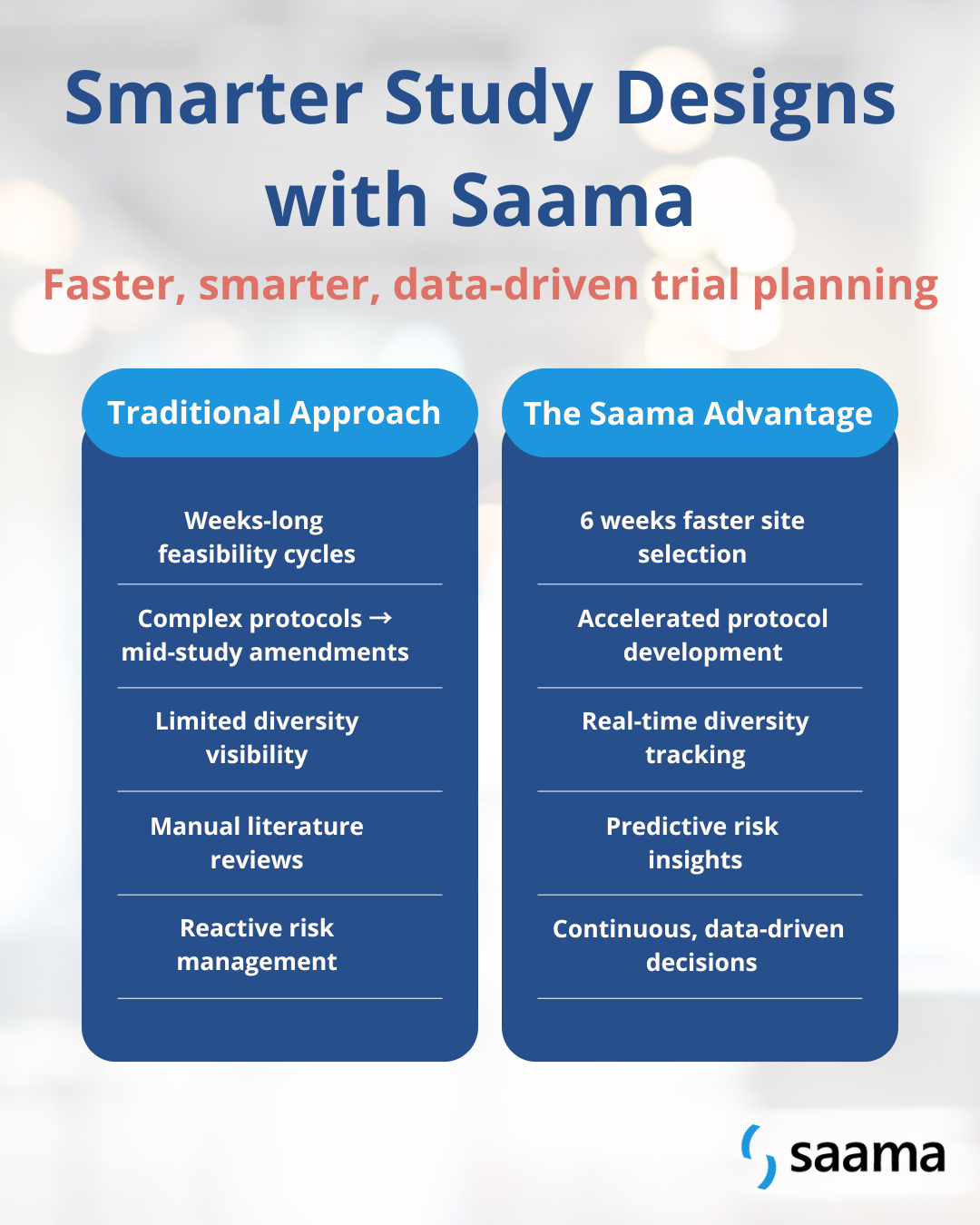
Running a trial isn’t just about choosing endpoints or patient groups. Teams need to make sure a clinical trial study is feasible, inclusive, and operationally smooth. Across the industry, common challenges include:
- Finding the right sites takes time: Multiple data sources make it complicated to know which sites can deliver results.
- Protocols are getting more complex: Modern therapies often need sophisticated designs to get meaningful results.
- Tracking diversity is difficult: Teams want studies to reflect real patients, but it’s not always easy to see enrollment trends in real time.
- Researching existing studies is slow: Gathering prior data to inform a new study can take weeks.
These are common industry realities. The question isn’t whether these challenges can be solved; it’s how to solve them faster, smarter, and with more confidence.
Below is a quick snapshot of how AI is reshaping study design from traditional challenges to measurable impact.
Three Ways AI is Making Trials Smarter
AI doesn’t replace expertise; it amplifies it. By handling routine work and surfacing insights, AI lets teams focus on big-picture decisions and patient-centered study design.
- Streamlined Site Selection:
Choosing the right clinical sites sets the foundation for study success. AI helps research teams make these decisions faster and with greater confidence. By bringing together data from multiple sources, including internal databases, CRO partners, and site performance history, AI creates a comprehensive view for decision-making. Teams can quickly identify and evaluate potential sites based on enrollment capabilities, regulatory efficiency, and investigator expertise. - Real-Time Diversity Insights:
Inclusive trials are both a scientific and regulatory priority. For instance: A large sponsor wanted to measure race, ethnicity, gender, and age across ongoing studies to ensure enrollment matched the patient population. Saama created an epidemiologic library linking this diversity data to real-time patient enrollment, giving the sponsor the ability to track progress and adjust strategies proactively. With these insights, trials became more representative and better aligned with patient needs. - Accelerated Literature Analysis:
Strong study designs build on existing scientific knowledge. AI transforms the literature review process from a time-intensive manual effort into an efficient, comprehensive analysis. AI-powered search tools can rapidly scan multiple databases, including clinical trial registries and published literature, to identify relevant studies.
Even better, these systems can automatically extract key information like patient populations, study designs, outcomes, and safety data. What previously required weeks of manual review can now be accomplished in days, giving research teams more time to focus on study design strategy and decision-making.
The Ripple Effect: Smarter Trials for Everyone
AI’s impact goes beyond speeding individual tasks:
- Faster site selection → studies start sooner
- Real-time diversity tracking → more representative trials
- Automated literature analysis → better-informed protocol design
By supporting research teams rather than replacing them, AI helps ensure studies are efficient, scientifically rigorous, and aligned with real-world patient needs.
Looking Ahead
Smarter trials start with smarter planning. With AI handling data, spotting risks, and uncovering insights, your team can focus on what matters most: designing studies that are faster, more inclusive, and truly patient-centered. Over time, trial planning could evolve into a more continuous, adaptive process where designs are not just smarter on day one, but stay smarter as new evidence emerges.
Discover how Saama’s AI solutions can help you design and deliver smarter trials: reach out to our experts at [email protected].
Frequently Asked Questions:
Q1. How does AI improve site selection in clinical trials?
A: AI analyzes historical performance, patient demographics, and investigator availability to identify optimal sites faster and more accurately.
Q2. How can AI support diversity in study design?
A: By leveraging real-world data, AI ensures inclusion of diverse populations across gender, age, and ethnicity for balanced clinical outcomes.
Q3. What are key steps to adopt AI in trial planning?
A: Define objectives, integrate data sources, validate models, and align stakeholders on AI-driven decision making.
